Each step is accompanied by explanations, templates, and examples. While the steps are ordered, this is an iterative process that should allow for each new design element to inform the previous and future parts. As you write, look backward and forward and make changes along the way. Use this example unit on flooding along with the planning templates for reference as you design.

The complexity of the natural world requires more than one core scientific concept to explain, and they often cross disciplinary boundaries. One example of this is the close relationship between the ideal gas law, a physical science concept, and its usefulness in explaining oxygen transport in the respiratory system.
Note that we aren’t suggesting starting with a three-dimensional learning goal. As mentioned in the project overview, we wanted to provide students with the agency to determine which science and engineering practices and crosscutting concepts they use to solve their challenges, rather than have them predetermined by the teacher.

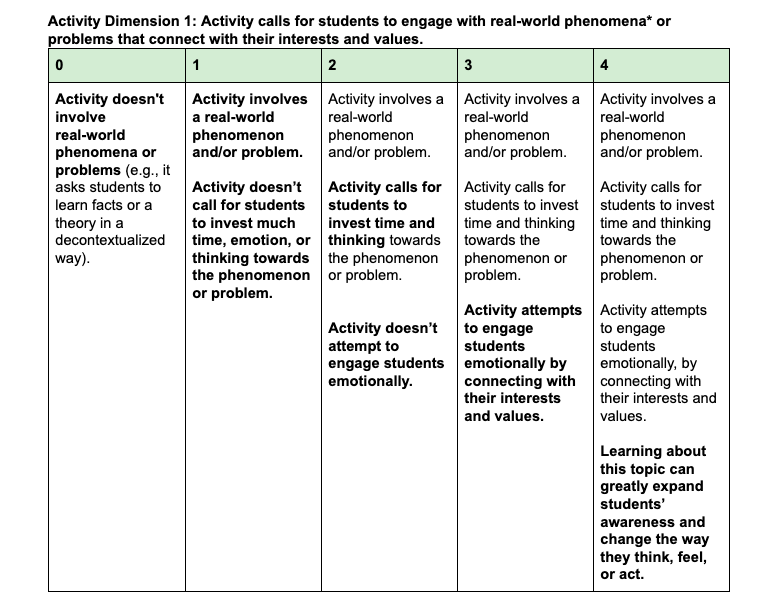
The best phenomena for a CBL project should inspire a call to action for students:
Because CBL is about solving community problems, this last point may seem difficult. How can we find local examples of all the phenomena needed to address every DCI? In this case, ask students to compare a broadly applicable phenomenon to their personal experience. For example, not all students may have experience with air pollution as severe as what occurs in Shanghai, but they can reflect on times when they have had to deal with smoke, allergens, car exhaust, or other causes of poor air quality. This resource from STEM Teaching Tools shares other characteristics of good phenomena.
For example, if we are designing around ESS3.C, and have chosen pollution of waterways due to electricity production as the phenomena, we might use the following explanation to create key understandings:
Coal is a natural product of organic matter being transformed over millions of years by heat and pressure in the Earth’s crust. As coal is formed, other chemicals in the crust like mercury, lead, and other heavy metals are stored along with the carbon. Coal is mined from the ground and burned by power plants to produce heat that turns water into steam, which is used to create electricity. When the coal is burned, the metal contaminants are released into the air, along with other chemicals like sulfur dioxide and nitrogen compounds. These chemicals become part of the atmosphere, and eventually find their way into the waterways and soil via the water cycle and precipitation. Once in soil or water, the contaminants remain in place because they are too heavy to evaporate along with the water as it moves through the water cycle.
With this explanation, we can identify some key understandings:

As you create key understandings, this is a good time to consider whether students might need an additional disciplinary core idea to provide a full explanation of the phenomena. Additionally, you should consider the depth of understanding a student needs based on the DCI progressions, and should not include concepts that are too far above or below their grade bands.

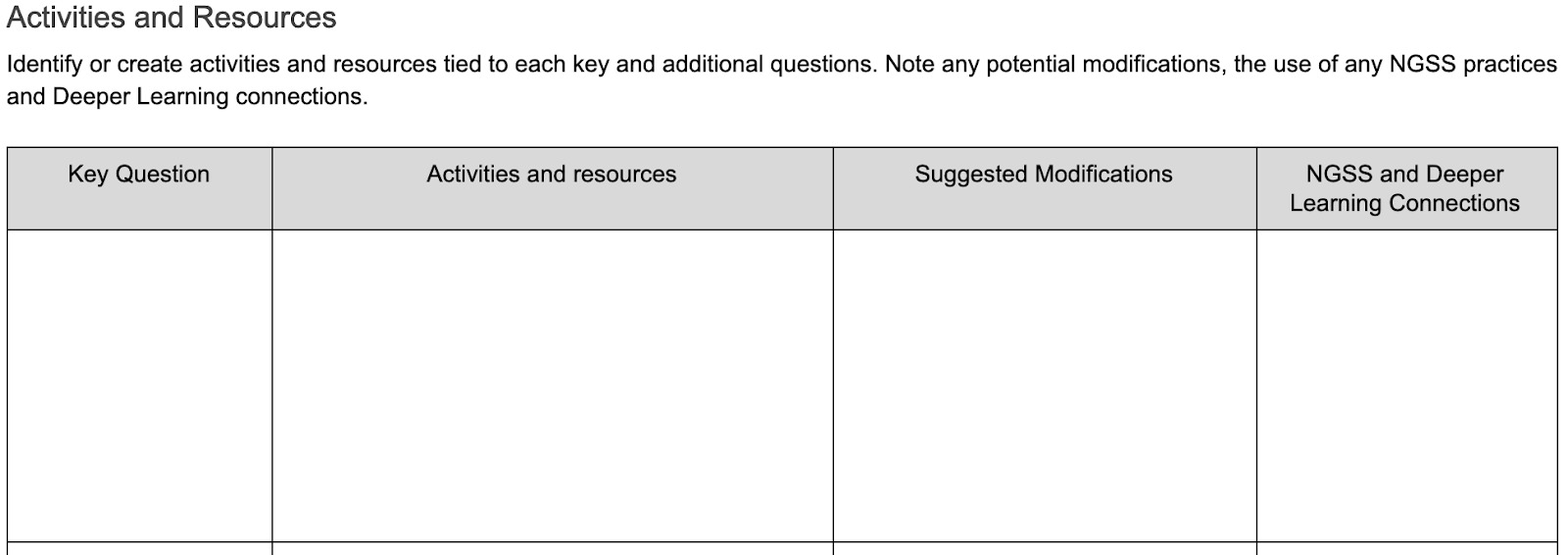
These questions will, in step 6, be divided between things students need to know to craft a high-quality challenge and things that students need to develop solutions. Note that these are potential student questions, and thought should be given to how to scaffold students into asking them. One strategy for promoting and organizing student questions is the driving question board.
Building on the question above, “Why doesn’t mercury or other metals evaporate out of the water?,” students could complete this NASA activity to get more information about and model evaporation. The resource could be slightly modified to place more emphasis on questioning and understanding why the salt is left behind, so that students can walk away with an understanding that the salt isn’t carried away because of its mass, and the mass of the metals is significantly higher, so they won’t be carried away either.
Continue to identify activities that will lead to answers to student questions for the rest of the key understandings, and add them to the Questions and Activities template. Identify and note the science and engineering practices and crosscutting concepts students will use during the activities, or make necessary modifications to include them. A variety of types of available activities will help students encounter multiple SEPs and CCCs in a single unit; the flexibility of OER, which are licensed to be free to use and modify, will assist in finding activities that are easily adaptable. Look for OER at OER Commons, the Library of Congress, federal agencies with educational outreach programs like NASA, or other state-led OER pages.
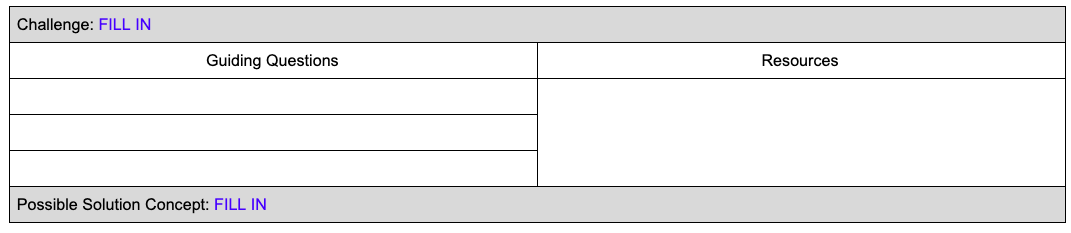
As you list the possibilities, consider what questions students might ask related to their challenge and what activities you could provide them that would help them answer those questions. Quality challenges are broad and actionable, but they do not contain a solution itself, allowing students multiple ways to address the problem and create diverse solutions. Using our water pollution example from above, a potential challenge might be: “Reduce heavy metals in our local waterways.” This challenge could result in a student solution that focuses on removing the existing contaminants from the water, while others might choose to address the soil. Another student might opt to try to stop the heavy metals being released at the source rather than waiting until they are already in the environment. Allowing for student choice increases engagement and ownership of learning, but requires flexibility and planning on the part of the teacher to identify what students need to know prior to creating a challenge and also what might need to be investigated by students to create a solution.

The two questions to ask yourself are:
Any questions and related activities that are necessary to craft a challenge are Essential Activities to be investigated up front during the Engage phase. Questions and activities that support understanding the phenomena in greater depth to develop a solution to the challenge are Guiding Questions and can be addressed in the Investigate phase. Using our pollution example from above, students might need to know where the contaminants come from and how they are released before they can craft a challenge. However, they may not need to know why the contaminants stay in the water and soil until they start investigating their potential solutions.
Using our pollution example above, the stakeholders might include a local office of the state’s natural resources division, people like fishermen and boaters who use the water as recreation, or a water department that collects and treats water for use in the community.


This work is licensed under a Creative Commons Attribution 4.0 International License.